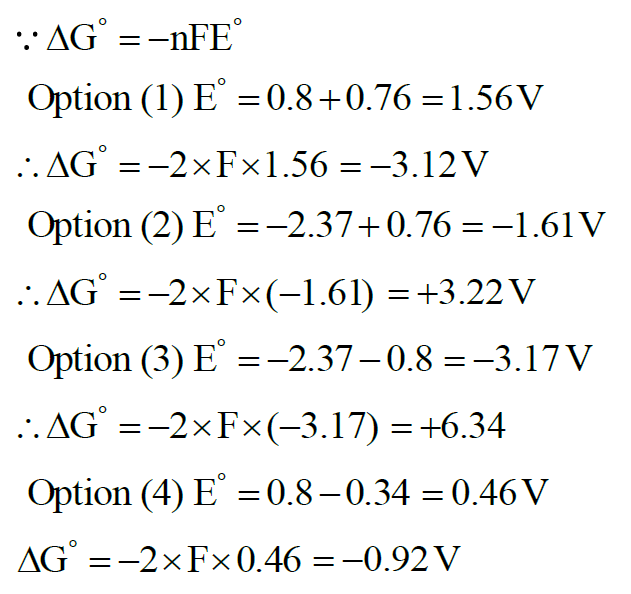
Standard electrode potentials for a few half cells are mentioned below :
$\mathrm{E}_{\mathrm{Cu}^{2+} / \mathrm{Cu}}^{\circ}=0.34 \mathrm{~V}, \mathrm{E}_{\mathrm{Zn}^{2+} / \mathrm{Zn}}^{\circ}=-0.76 \mathrm{~V}$
$\mathrm{E}_{\mathrm{Ag}^{+} / \mathrm{Ag}}^{0}=0.80 \mathrm{~V}, \mathrm{E}_{\mathrm{Mg}^{2+} / \mathrm{Mg}}^{0}=-2.37 \mathrm{~V}$
Which one of the following cells gives the most negative value of $\Delta \mathrm{G}^{\circ}$ ?