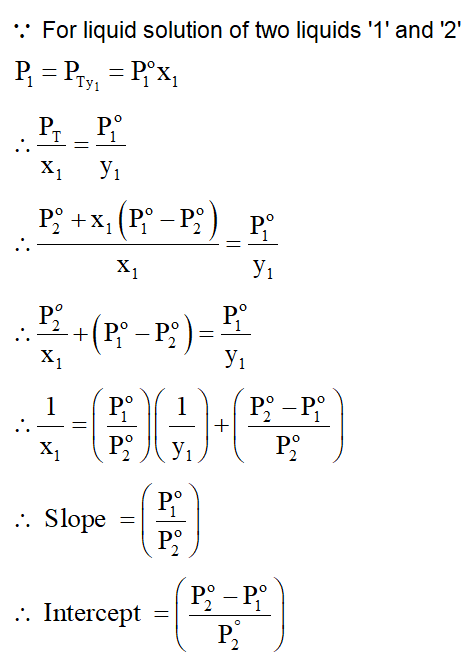Consider a binary solution of two volatile liquid components 1 and $2 . x_{1}$ and $y_{1}$ are the mole fractions of component 1 in liquid and vapour phase, respectively. The slope and intercept of the linear plot of $\frac{1}{x_{1}}$ vs $\frac{1}{y_{1}}$ are given respectively as :